Für Unternehmen
Strom & Gas Management
Mehrwert durch maßgeschneiderte Lösungen! Nutzen Sie den Vorteil von 85 Anbietern und sichern Sie sich herausragenden Service, passende Tarife und konkurrenzlose Preise.
Nutzen Sie den Vorteil intelligenter Energiebeschaffung. Denn wahre Wirtschaftlichkeit verbirgt sich oft hinter mehr als NUR einem scheinbar niedrigen Preis.
Was macht OPTUM zur ersten Wahl?
Premium für jedes Unternehmen
Wir sichern Ihnen Top-Konditionen und erstklassigen Service. Denn echter Wert geht über den reinen Preis hinaus.
Keine Überraschungen, nur Klarheit
Transparente Preise, wie versprochen. Weil günstige Energie mehr ist als ein günstiges Angebot.
Kontinuierlich günstige Energie durch Expertise
Dank unserer Marktkenner und OPTUM PRO sichern wir den idealen Einkaufszeitpunkt für Strom und Gas – für Ihre dauerhaft besten Konditionen.
Vorausschauende Versorgungssicherheit
Durch unser breites Lieferantenportfolio erkennen wir potenzielle Engpässe am Markt frühzeitig – für Ihre ununterbrochene Energieversorgung.
Maßgeschneidert
Unabhängige Branchenexpertise speziell für produzierende Unternehmen.
Anforderungen an unsere Kunden
Bei OPTUM EBA verstehen wir, dass eine effiziente und zuverlässige Energieversorgung für Unternehmen mit hohem Energiebedarf entscheidend ist. Daher konzentrieren wir uns darauf, unseren maßgeschneiderten und erstklassigen Service speziell für Unternehmen anzubieten, die eine jährliche Energieabnahme von über 20 Gigawattstunden (GWh) haben.
Diese spezifische Ausrichtung ermöglicht es uns, zielgerichtete und hochwertige Energielösungen bereitzustellen, die optimal auf die komplexen Bedürfnisse und Herausforderungen von Großverbrauchern abgestimmt sind.
Warum diese Fokussierung wichtig ist:
Spezialisierte Lösungen
Effizienzsteigerung
Risikomanagement
Nachhaltige Energiepartnerschaften
Unser Engagement für Großverbraucher
Unser Engagement für Großverbraucher
OPTUM EBA ist bestrebt, führend in der Bereitstellung von hochwertigen, zuverlässigen und maßgeschneiderten Energielösungen für Großverbraucher zu sein. Wir verstehen, dass jedes Unternehmen einzigartig ist, und sind hier, um sicherzustellen, dass Ihre spezifischen Energiebedürfnisse effizient und effektiv erfüllt werden.
Mit unserem Fokus auf Unternehmen, die mehr als 20 GWh pro Jahr verbrauchen, setzen wir uns dafür ein, dass Ihre Energieversorgung nicht nur eine Notwendigkeit, sondern ein strategischer Vorteil für Ihr Unternehmen ist.
In 4 Schritten zu dauerhaft niedrigen Energiekosten mit OPTUM EBA
Ist-Analyse – Ihr aktueller Standpunkt?
Nutzen Sie die Gelegenheit für ein Expertengespräch und lassen Sie sich von unserem Team unverbindlich beraten. Wir prüfen Ihren Vertragsstatus, beleuchten technische Aspekte und werten Ihre Abrechnungsdaten aus, um Ihren spezifischen Bedarf klarzustellen.
Potenzialermittlung – Wo liegen Ihre Möglichkeiten?
Unter Berücksichtigung Ihres Energieverbrauchs, der Energiekosten, des Lastgangs und der aktuellen Börsenpreise für Energie führen wir eine maßgeschneiderte Potenzialanalyse für Sie durch.
Energievertrag optimal gestalten
Wir verhandeln intensiv mit den Energielieferanten, immer mit Ihrem besten Interesse im Blick. Den endgültigen Vertrag unterzeichnen Sie dann unter optimalen Bedingungen direkt mit dem passenden Lieferanten.
Kontinuierliche Betreuung und Marktbeobachtung mit OPTUM EBA
Auch nach der Vertragsunterzeichnung lassen wir Sie nicht allein. Bei Fragen oder Herausforderungen rund um die Energiebeschaffung steht Ihnen unser Expertenteam stets zur Seite. Zudem beginnen wir mit der fortwährenden Marktbeobachtung, um für Sie vorausschauend und zum optimalen Zeitpunkt in den kommenden Jahren einzukaufen.
Expertendialog
Unser Ziel ist es, genau zu erfassen, wo Sie momentan stehen, welche Ziele Sie verfolgen, welche Maßnahmen bereits umgesetzt wurden und welche noch in der Planung sind. Nur so können wir effektiv herausfinden, wie wir Sie optimal unterstützen können.
Aktueller Chart für Strom am Spotmarkt
Aktuelle Großhandelspreise für Strom in Europa
Eine Haftung der OPTUM für die Richtigkeit und Vollständigkeit der Daten wird ausgeschlossen.
Quelle: Bundesnetzagentur I SMARD.de
Gewichteter Stromspothandelspreis
(Day-Ahead-Börsenstrompreis) je
Stunde |€/MWh] resultierend aus der vortägigen Day-Ahead-Auktion - Datenlieferung erfolgt spätestens 48 Stunden nach Handelsschluss.
Navigieren Sie mit Expertise vom klassischen Terminmarkt hin zu innovativen Großhandelskonditionen am Spotmarkt.
Spotmarkt-Trends: Der Wandel des Marktes verlangt ständige Anpassungen. Wo einst Festpreisverträge dominierten, bieten 2023 alternative Energiebeschaffungsmethoden sowohl Kosteneffizienz als auch Flexibilität – vor allem in Phasen, in denen der Spotmarkt dank Preisbremsen und ohne Risikoaufschläge dem Terminmarkt überlegen ist.
Spotmarkt-Ausschreibungen: Großhandelspreise und maßgeschneiderte Konditionen.
Mit zielgerichteten Ausschreibungen am Spotmarkt bietet Ihr OPTUM Agent Großkunden den Zugang zu den standardisierten Börsenpreisen, ergänzt durch exzellente Vertragsoptionen und Servicegebühren. Unsere Branchenkenntnisse schaffen signifikante Einsparmöglichkeiten in Ihrer Vertragsausgestaltung. Dank sorgfältig geplanter Einkaufsstrategien sind Sie immer vorn dabei. Mit unseren hybriden Tarifen, die Spot- und Terminmarkt kombinieren, gewährleisten wir Ihnen die optimale Balance von Kosteneffizienz und Flexibilität. In unsicheren Marktlagen stellt unsere Switchoption einen reibungslosen Wechsel zum Terminmarkt sicher und garantiert so Ihre Preiskonsistenz.
Spitzenkompetenz im Markt
In der vielschichtigen Energiebranche zählt das Detailwissen. Vertrauen Sie auf uns, von der Strategie bis zur Umsetzung. Ihr dedizierter Ansprechpartner bei OPTUM EBA sorgt dafür, dass keine Frage unbeantwortet bleibt und Sie sich vollends Ihrem Geschäftsfeld widmen können.
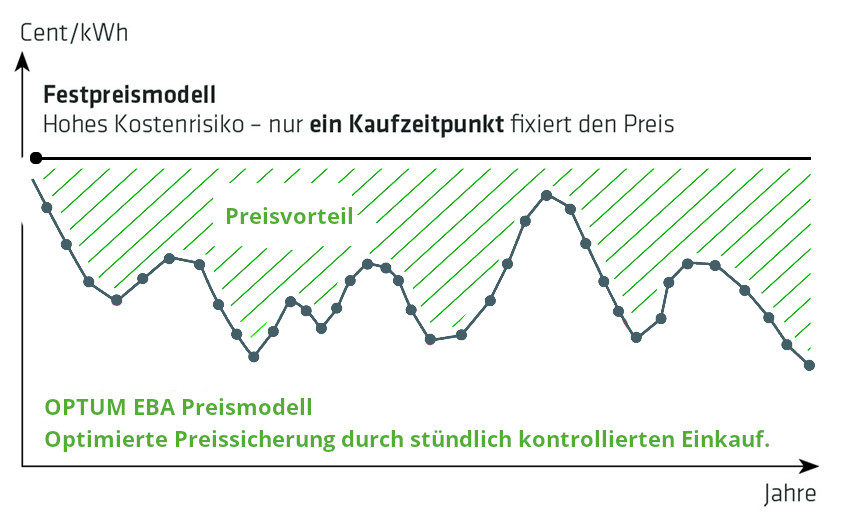
Festpreis vs. Variabler Preis Risiken und Chancen in der Energiebeschaffung
Die Grafik verdeutlicht: Bei Festpreisverträgen wird der Energiepreis beim Kauf fixiert, ohne Anpassung während der Laufzeit – ein potenzielles Kostenrisiko bei sinkenden Marktpreisen. Variable Verträge dagegen passen sich den Marktpreisen an und können so Kosteneffizienz steigern. Die Wahl zwischen Fix- und Variabelverträgen erfordert eine strategische Abwägung, um das Kostenrisiko zu steuern und die Beschaffung zu optimieren.
OPTUM
Maßgeschneiderte Energiebeschaffung mit Weitblick
Dank unserer Prognosen konnten viele unserer Mandanten bis Ende 2024/2025 von festen Preisen profitieren und aktuelle Marktentwicklungen gelassen beobachten. Schon 2021 warnten wir vor steigenden Energiepreisen und halfen zahlreichen Kunden, nicht in die Falle kostspieliger Verträge zu tappen. Unsere Voraussicht lässt sich anhand der Zeitstempel unserer Social-Media-Veröffentlichungen nachvollziehen
In der volatilen Welt des Energiemarktes versäumen es viele Unternehmen, ihre Beschaffungsstrategien anzupassen, und lassen nicht nur Einsparpotenzial liegen, sondern bringen sich auch in existenzbedrohende Situationen. Angesichts der Komplexität des Marktes fehlt vielen die nötige Zeit und das Fachwissen. Mit unserer Expertise in der intelligenten Energiebeschaffung setzen wir genau hier an und sichern Ihren unternehmerischen Vorteil.
OPTUM PRO: Ihr Navigator durch Daten- und KI-gesteuerte Präzision im Energiemarkt
Unser hochentwickeltes Analysesystem, OPTUM PRO, verschafft unseren Experten einen fundierten Einblick in die Feinheiten des Energiemarktes. Mit der Verschmelzung von daten- und KI-gestützten Vorhersagen und spezialisierten Lieferantenalgorithmen, die akkurat die Angebote von Anbieterausschreibungen auswerten, erhalten wir essenzielle Informationen über Marktverhaltensmuster. In Kombination mit einem gründlichen Marktvergleich sind wir in der Lage, den idealen Zeitpunkt für Energiekäufe mit hoher Wahrscheinlichkeit zu bestimmen. Auf Basis dieser Expertise verfeinern wir Laufzeiten und Beschaffungsstrategien effektiver als unsere Mitbewerber, wodurch wir Ihnen kontinuierliche Kostenvorteile bieten.
Mit OPTUM PRO haben wir deshalb ein Analysesystem entwickelt, mit dem wir durch datenbasierte Vorhersagen, intelligente Lieferantenalgorithmen und einen systematischen Marktvergleich den jeweils besten Energieliefervertrag identifizieren können.
Auf Basis dieser Daten handeln wir dann die neuen Strom- und Gasverträge aus, mit denen Sie ab sofort dauerhaft bares Geld sparen!
Smart Metering trifft Spotmarkt-Expertise.
Energieeffizienz auf Höchstniveau: Intelligente Messtechnik trifft auf Spotmarkttarife.
Smart Meter definieren die Energieüberwachung neu. Mit ihrer Hilfe lässt sich der Strom- und Gasverbrauch in Echtzeit erfassen, wodurch proaktive Interventionen bei jeder Art von Energieineffizienz möglich werden. Spezialisierte Analysetools bieten eine tiefgehende Inspektion Ihrer Energiekosten, sodass Verbrauchsmuster optimiert und signifikante Effizienzsteigerungen erreicht werden können.
Der Spotmarkt für Strom und Gas steht für beispiellose Transparenz und Flexibilität. Er erlaubt es, antizipierend und strategisch auf Marktveränderungen zu antworten und Energie zu den optimalsten Zeiten zu beziehen.
Selbst bei Zählersystemen ohne integrierte Smart Meter-Technologie bieten unsere digitalen Ablesesysteme eine klare und intuitiv nachvollziehbare Darstellung des Energieverbrauchs. So können Sie potenzielle Ineffizienzen frühzeitig identifizieren und zielgerichtete Anpassungen vornehmen, um Ihre Energieversorgung zu optimieren.
Setzen Sie auf unser Expertenteam, das durch erstklassige Branchenkenntnisse und weitreichende Erfahrung überzeugt.
Expertendialog
Wir nehmen uns die Zeit, Ihre aktuelle Position, die bereits umgesetzten Maßnahmen sowie Ihre zukünftigen Vorhaben und Ziele im Detail zu verstehen. Auf dieser Grundlage erarbeiten wir, wie wir Sie am besten unterstützen und begleiten können.
OPTUM: Ein verlässlicher Partner seit 20 Jahren
Seit 2003 fungiert OPTUM als Unternehmensberater im Personalbereich, begleitet Unternehmen bei der Bildung leistungsstarker Teams und bietet sowohl die Vermittlung qualifizierter Fachkräfte als auch fundierte Beratung zu Personalfragen.
Nach 13 wunderbaren Jahren im Personaldienstleistungsbereich haben wir uns entschieden, für unsere Kunden eine neue Tür zu öffnen: Den Zugang zu Strom- & Gas-Ausschreibungen für Großkunden.
Interessante Blogbeiträge
Häufig gestellte Fragen
Wie kann ein Gewerbestromvergleich dabei helfen, den besten Stromanbieter für Unternehmen zu finden?
Unternehmen auf der Suche nach dem besten Strompreis sollten unbedingt einen Gewerbestromvergleich durchführen. Ein solcher Vergleich ermöglicht es Unternehmen, die verschiedenen Angebote von Stromanbietern zu vergleichen und das beste Angebot für ihre Bedürfnisse zu finden.
Ein wichtiger Faktor bei der Wahl des richtigen Stromanbieters ist die Art des Vertrags. Es gibt verschiedene Arten von Stromverträgen, wie zum Beispiel feste Preise, variable Preise und grünen Strom. Unternehmen sollten sich über die verschiedenen Optionen informieren und das Angebot wählen, das am besten zu ihren Anforderungen passt.
Ein weiterer wichtiger Aspekt bei der Wahl des richtigen Stromanbieters ist der Preis. Ein Unternehmen Preisvergleich kann helfen, die verschiedenen Preise der Anbieter zu vergleichen und das beste Angebot zu finden. Es ist jedoch wichtig zu beachten, dass der günstigste Preis nicht immer der beste Weg ist, da ein günstiger Preis oft mit einer schlechteren Servicequalität oder weniger flexiblen Vertragsbedingungen einhergeht.
Eine weitere Möglichkeit Unternehmen bei der Wahl des richtigen Stromanbieters unterstützt zu werden, ist die Inanspruchnahme von professioneller Hilfe.
OPTUM EBA führt Gewerbestromvergleiche für den Kunden durch und hilft bei der Auswahl des besten Angebots.
Wir haben bereits vielen Kunden in diesem Thema geholfen und können Ihr Unternehmen auch dabei unterstützen, die beste Wahl für ihre Bedürfnisse zu treffen.
Welchen Aufwand habe ich mit der Beschaffung am Spotmarkt?
Die Energiebeschaffung über den Spotmarkt erfolgt in der Regel über einen Spotmarktlieferanten, der sich um die gesamte energiewirtschaftliche Abwicklung kümmert. Sie müssen sich also um nichts kümmern und können sich ganz auf Ihr Kerngeschäft konzentrieren.
Erhalte ich dann Ökostrom?
.Ja, bei der Teilnahme an Ausschreibungen für Strom am Spotmarkt haben Sie die Möglichkeit, auch Ökostrom zu beziehen. Es ist jedoch wichtig, diese Option bei der Teilnahme an der Ausschreibung spezifisch anzufordern.
Was ist ein Lastgang?
Ein Lastgangprofil ist eine Aufstellung der Strommenge, die von einem Verbraucher über einen bestimmten Zeitraum hinweg verbraucht wurde. Es zeigt also den Verlauf des Stromverbrauchs über einen bestimmten Zeitraum an. Lastgangprofile werden häufig von Energieversorgungsunternehmen und Industriebetrieben erstellt, um den Stromverbrauch zu analysieren und zu optimieren. Sie können auch als Basis für die Gestaltung von Energieverträgen dienen.
Ab einem Jahresverbrauch von etwa 100.000 kWh Strom oder 1.5 Millionen kWh Gas werden RLM-Zähler (Registrierende Leistungsmessung) verwendet. Diese Geräte erfassen pro Messzeitraum (15 Minuten bei Strom, 60 Minuten bei Gas) den Leistungsmittelwert und übermitteln diese Daten regelmäßig an den Netzbetreiber, der sie dann an den Stromanbieter weiterleitet. RLM-Zähler berechnen jeden Monat die tatsächliche Leistung und den daraus resultierenden Verbrauch. In Zahlen für Strom ausgedrückt sind das 2.880 Leistungswerte pro Monat (30 Tage x 24 Stunden pro Tag x 4 Leistungswerte pro Stunde). Alle Werte eines Jahres ergeben den sogenannten Jahreslastgang.“
Gibt es am Spotmarkt Risikoaufschläge?
Im Gegensatz zu herkömmlichen Stromtarifen mit einem Festpreis pro Kilowattstunde ist ein Spotmarkttarif dynamisch. Das heißt, Sie profitieren von minimierten Risikoaufschlägen, die ansonsten im Preis einkalkuliert wären.
Was kostet Strom an der Börse?
Der Preis für Strom an der Börse hängt von verschiedenen Faktoren ab, wie zum Beispiel dem Angebot und der Nachfrage, den Wetterbedingungen und der Verfügbarkeit von Energieerzeugungsanlagen. Der Preis kann daher täglich schwanken und ist nicht im Voraus festlegbar. Chart für Spotmarkt Strom
Wie funktioniert der Energiehandel am Spotmarkt?
Der Energiehandel am Spotmarkt erfolgt über die Spotmarktbörse, auf der Käufer und Verkäufer von Energie zusammenkommen. Die Preise werden hier täglich festgelegt und es werden Energiemengen für den nächsten Tag oder für einen späteren Zeitpunkt gehandelt. Unternehmen, die Energie am Spotmarkt einkaufen, profitieren von tagesaktuellen Preisen und haben so die Möglichkeit, Ersparnisse und Optimierung zu erzielen.
Wie entstehen die Strompreise?
Die Strompreise entstehen durch den interaktiven Prozess zwischen Angebot und Nachfrage auf dem Energiemarkt. Dabei spielen verschiedene Faktoren wie die Verfügbarkeit und Kosten von Energieerzeugungskapazitäten, die Wetterbedingungen und die politischen Rahmenbedingungen eine Rolle. Auf der Angebotsseite gibt es verschiedene Energieerzeugungsformen wie fossil befeuerten Kraftwerke, Kernenergie, erneuerbare Energien und andere, die zu unterschiedlichen Preisen Strom bereitstellen. Auf der Nachfrageseite gibt es Industrieunternehmen, Haushalte und andere, die Strom konsumieren und bereit sind, dafür zu bezahlen. Der Preis für Strom wird letztendlich durch den Gleichgewichtspunkt zwischen Angebot und Nachfrage bestimmt.
Wer darf an der Strombörse handeln?
An der Strombörse dürfen sowohl private Unternehmen als auch öffentliche Einrichtungen handeln. Es gibt jedoch bestimmte Voraussetzungen, die erfüllt sein müssen, um an der Börse handeln zu dürfen, wie zum Beispiel eine entsprechende Zulassung und die Einhaltung von Regelungen und Vorschriften.
Welche Vorteile habe ich mit Spotmarkttarifen ?
Spotmarkttarife bieten in der Regel einige Vorteile im Vergleich zu Festpreisverträgen:
- Dynamische Preise: Der Preis für Energie am Spotmarkt wird täglich angepasst, um dem aktuellen Angebot und der Nachfrage gerecht zu werden. Dadurch haben Sie die Möglichkeit, von günstigen Preisen zu profitieren, wenn sie verfügbar sind.
- Geringere Risikoaufschläge: Festpreisverträge enthalten häufig Risikoaufschläge, um das Risiko von Preisschwankungen abzufedern. Spotmarkttarife haben diese Aufschläge nicht, da der Preis dynamisch angepasst wird.
- Flexibilität: Spotmarkttarife bieten Ihnen die Möglichkeit, von täglich ändernden Preisen zu profitieren. Sie haben somit mehr Flexibilität und Kontrolle über Ihre Energiebeschaffung.
- Einfache Abwicklung: Die Energiebeschaffung über den Spotmarkt erfolgt in der Regel über einen Spotmarktlieferanten, der sich um die gesamte energiewirtschaftliche Abwicklung kümmert. Sie müssen sich also um nichts kümmern und können sich ganz auf Ihr Kerngeschäft konzentrieren.
Was mache ich, wenn sich der Markt wieder dreht?
Sollte sich der Markt ändern und die Bedingungen sich drehen, ist es ratsam, auf einen hybriden Tarif zurückzugreifen, der sowohl die Bedingungen des Spotmarktes als auch des Terminmarktes berücksichtigt. Dies bietet in solchen Fällen Flexibilität und Kostenkontrolle. Es ist wichtig, den Markt regelmäßig zu beobachten und das Timing sorgfältig abzuwägen, um die bestmögliche Entscheidung zum richtigen Zeitpunkt treffen zu können. Um bei dieser Entscheidung unterstützt zu werden, ist es sinnvoll, sich professionelle Hilfe von Energieexperten wie die von OPTUM EBA zu holen, die sich um die Energiebeschaffung kümmern und Sie unabhängig vom Lieferanten beraten.
Was sind die Vorteile der Energiebeschaffung mit OPTUM EBA für Geschäftskunden?
Als Geschäftskunde ist es wichtig, den passenden Energieversorger und den günstigsten Tarif für Strom und Gas zu finden. Denn ein hoher Energieverbrauch kann eine große Belastung für das Unternehmensbudget darstellen. Mit OPTUM EBA bietet sich eine effiziente Lösung für die Energiebeschaffung, die auf die Bedürfnisse von Geschäftskunden abgestimmt ist.
Welche Vorteile bieten spezielle Tarife und Vertragsbedingungen für Unternehmen mit hohem Strom- oder Gasverbrauch?
Für Unternehmen mit einem hohen Strom- oder Gasverbrauch gibt es spezielle Tarife und Vertragsbedingungen. Diese sind auf den besonders hohen Verbrauch abgestimmt und können sich durch günstigere Preise, flexible Lieferbedingungen oder individuelle Serviceleistungen auszeichnen. Mit OPTUM EBA können Geschäftskunden die verschiedenen Angebote von verschiedenen Anbietern einfach und schnell vergleichen und so den für sie besten Tarif finden.
Wie können Großkunden und die Industrie mit OPTUM EBA den besten Preis für Erdgas finden?
Erdgasbeschaffung für Großkunden und die Industrie
Die Erdgasbeschaffung ist insbesondere für die Industrie von großer Bedeutung, da sie häufig große Mengen an Erdgas benötigt. Unternehmen, die ihr Erdgas am Spotmarkt einkaufen, profitieren von tagesaktuellen Preisen und haben so die Möglichkeit, Ersparnisse und Optimierung zu erzielen. Mit OPTUM EBA können Großkunden und die Industrie den Börsenpreis für Erdgas verfolgen und die Angebote von verschiedenen Anbietern vergleichen, um den besten Preis für ihr Erdgas zu finden.
Wie können Geschäftskunden mit einem Stromverbrauch größer als 100.000 kWh ihre Einsparpotentiale identifizieren?
Unternehmen mit einem Stromverbrauch größer 100.000 kWh pro Jahr gelten als Großkunden und können von speziellen Tarifen und Vertragsbedingungen profitieren. Mit OPTUM EBA können diese Unternehmen ihren Stromverbrauch überwachen und gegebenenfalls Maßnahmen zur Optimierung ergreifen, um Einsparpotentiale zu identifizieren.
Wie können Geschäftskunden mit einem Erdgasverbrauch größer als 1,5 Millionen Kilowattstunden (kWh) ihre Einsparpotentiale identifizieren?
Es gibt mehrere Möglichkeiten, wie Geschäftskunden mit einem Erdgasverbrauch von mehr als 1,5 Millionen Kilowattstunden (kWh) ihre Einsparpotentiale identifizieren können:
Energieaudit:
Ein Energieaudit ist eine umfassende Analyse des Energieverbrauchs und der Energieeffizienz eines Unternehmens. Mit Hilfe eines Energieaudits können Einsparpotentiale identifiziert und Maßnahmen zur Energieeinsparung und -effizienzsteigerung entwickelt werden.
Verbrauchsmessung:
Eine genaue Verbrauchsmessung kann dazu beitragen, den Gasverbrauch und die Verbrauchsverteilung im Unternehmen zu erfassen und gezielt Einsparungen zu erzielen. Dabei können beispielsweise Einzelverbraucher erfasst und Verbrauchsspitzen aufgedeckt werden.
Beratung durch Energieexperten:
Energieexperten können dabei helfen, Einsparpotentiale zu identifizieren und Maßnahmen zur Verringerung des Gasverbrauchs zu entwickeln. Sie können auch bei der Umsetzung von Energieeinsparmaßnahmen unterstützen und die Wirtschaftlichkeit solcher Maßnahmen bewerten.
Energiemanagementsystem: Ein Energiemanagementsystem (EMS) ist ein organisatorisches und technisches Konzept, das darauf abzielt, den Energieverbrauch und die Energieeffizienz eines Unternehmens zu optimieren. Im Rahmen eines EMS können Ziele zur Energieeinsparung festgelegt und Maßnahmen zur Erreichung dieser Ziele ergriffen werden.
Nutzung von Energieeffizienzmaßnahmen:
Es gibt viele Möglichkeiten, den Gasverbrauch durch den Einsatz von Energieeffizienzmaßnahmen zu reduzieren. Dazu gehören beispielsweise der Einsatz von Energieeffizienzpumpen, die Dämmung von Gebäuden oder die Optimierung der Prozesse im Unternehmen. Eine ausführliche Überprüfung und Bewertung solcher Maßnahmen kann dabei helfen, das Einsparpotential zu ermitteln und gezielt Einsparungen zu erzielen
Was ist das Tranchenmodell bei der strukturierten Beschaffung von Strom und Gas und wie funktioniert es?
Durch die Einteilung in Tranchen können diese Unternehmen ihren Energiebedarf langfristig planen und sich somit auf die Zukunft vorbereiten. Durch den Vergleich von Angeboten verschiedener Energieversorger können sie zudem den besten Preis für ihren Strom und Gas finden. Auch für Energieversorger bietet das Tranchenmodell Vorteile, da es ihnen eine gewisse Planungssicherheit gibt und sie ihre Kapazitäten besser auslasten können.
Insgesamt bietet das Tranchenmodell somit eine effektive Möglichkeit, die Energiebeschaffung für Unternehmen und öffentliche Einrichtungen zu optimieren.
Was kostet mich das?
Was kostet mich die Energiebeschaffung über den Spotmarkt?
Sie zahlen den aktuellen Gas & Strompreis an der Börse und zusätzlich eine Servicegebühr, die von Ihrem Lieferanten festgelegt und Ihnen transparent mitgeteilt wird. Diese Gebühr deckt die Kosten für den Energieeinkauf, Vertrieb und Verwaltung ab.
Welche Faktoren beeinflussen die Kosten für professionelle Hilfe bei der Energiebeschaffung?
Es ist schwierig, einen genauen Preis für professionelle Hilfe bei der Energiebeschaffung anzugeben, da die Kosten von verschiedenen Faktoren wie dem Umfang der Dienstleistungen, der Größe und dem Energiebedarf des Unternehmens und eventuellen individuellen Anforderungen abhängen.